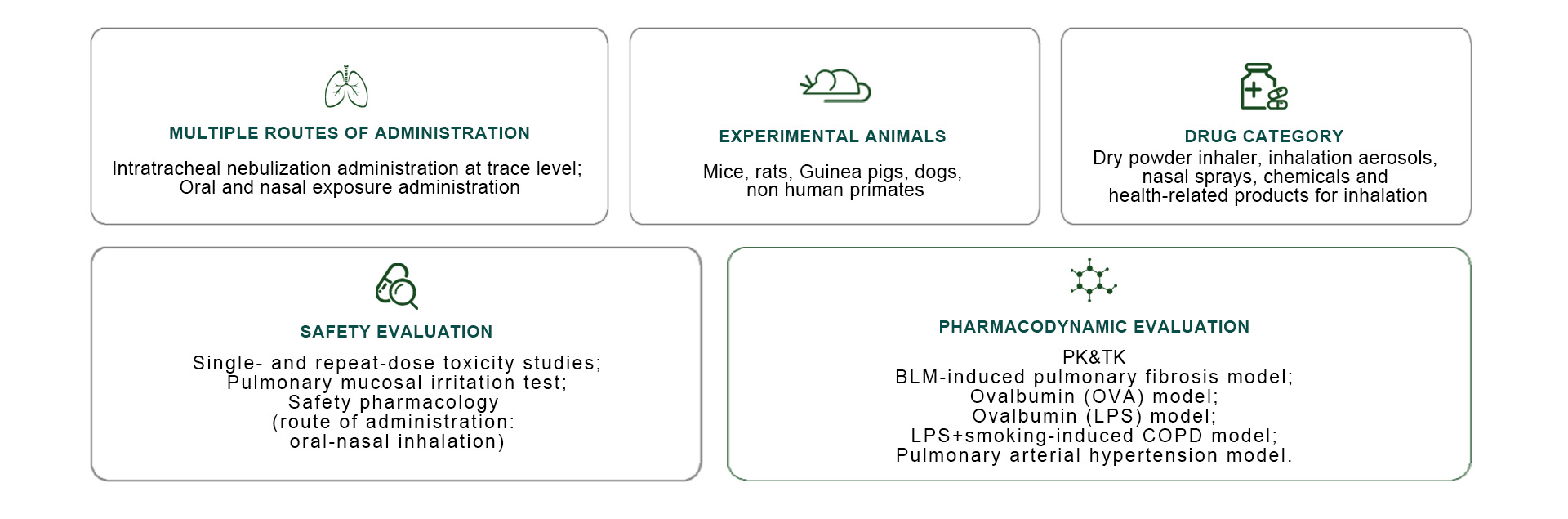
WestChina-Frontier has established an internationally leading inhalation formulation research platform. Equipped with advanced instruments (e.g., inhalation exposure system, airway atomization device, precision inhalation system) for exposing large and/or small animals to inhalation drugs, coupled with pathological examination of the respiratory system, lung function tests, and biochemical cytological evaluation of pulmonary perfusion, We can provide customers with non-clinical evaluation services for inhaled drugs at all stages of inhaled dosage form research that fully comply with NMPA/FDA/OECD regulations requirements for every phase of the study of inhalation formulations in a comprehensive way. The platform boasts a professional team of more than 20 experienced non-clinical researchers, who have the ability to complete the PD, PK and toxicology studies of inhalation solutions, dry powder inhaler, inhalation aerosols, soft mist inhalers, nasal sprays and other inhalation-related products in mice, rats, Guinea pigs, dogs, and monkeys.


Business Contacts:028-60662528
E-mail:service@glpcd.com
+8628-60662518-8800
+8628-85154334-800
