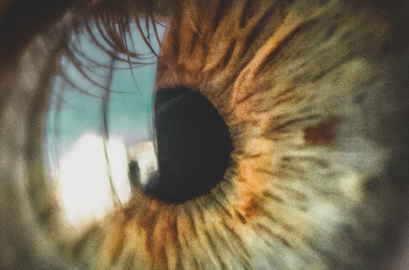
Founded in 2006, the Evaluation of Ophthalmic Drugs has so far emerged as a first-class non-clinical ophthalmology business division in China. It boasts a team of experienced professionals in ophthalmology and possesses complete, internationally-advanced ophthalmic testing and operational equipment. With the capability to carry out various non-clinical studies of ophthalmic drugs of diverse dosage forms and routes of administration, it stands as a notable player in the field. One of its representative marketed drug is conbercept®.
MORE
WestChina-Frontier has constructed an inhalation drug R&D platform supported by a professional team dedicated to the evaluation and development of inhalation drugs. Equipped with Chinese and foreign advanced technology and equipment, the platform provides clients with all-round professional technical services covering early drug screening, pharmacodynamic study, PK study, and safety evaluation.</p>
MORE
WestChina-Frontier has established a team of professionals qualified for the use of more than 30 radioactive isotopes to label study drugs; the team has the capability to provide PET molecular imaging and pre-clinical evaluation of PK, efficacy and safety of radioactive isotope labeled drugs. WestChina-Frontier has equipped itself with animal experiment facilities for the study of radioactive isotope labeled drugs, drug safety evaluation laboratories, molecular imaging laboratories, PET/CT, and a complete set of large equipment for drug safety evaluation. We also have purchased complete sample treatment and testing equipment in order to provide non-clinical safety evaluation and molecular imaging study of radioactive isotope labeled drugs.
MORE
WestChina-Frontier has established a GLP-compliant mature technical system for evaluation of drug dependence, a platform for pharmacodynamic study of analgesics, and an integrated technology platform to monitor the cardio-pulmonary functions and ECG of large animals on anesthetics.
MORE
WestChina-Frontier has constructed a comprehensive study system for pediatric drugs, having completed the evaluation of more than 50 pediatric drugs in juvenile animals (the experimental animal species include: neonate rats, neonate rabbits, juvenile pigs, juvenile dogs); the evaluated pediatric drugs are indicated for hand-foot-and-mouth disease, infantile eczema, pediatric myopia, precocious puberty, anti-tumor, neonatal jaundice, skin haemangioma, psoriasis, etc.
MORE

Business Contacts:028-60662528
E-mail:service@glpcd.com
+8628-60662518-8800
+8628-85154334-800
