WestChina-Frontier's safety pharmacology team consists of China’s national certified veterinarian and toxicologists. All members of the team are skilled, experienced, and possess the education background in clinical veterinary medicine, pharmacology and clinical medicine.
• Perfect safety pharmacological study system for safety pharmacological study and regulatory submission of new drugs in line with ICH guidelines, NMPA/FDA/OECD regulatory requirements and GLP.
• All equipment used for core safety pharmacological studies meets the requirements specified in 21 CFR Part 11 on electronic data.
• Partial exploratory safety pharmacological research service (Non-GLP safety pharmacology screening study) provided.
•
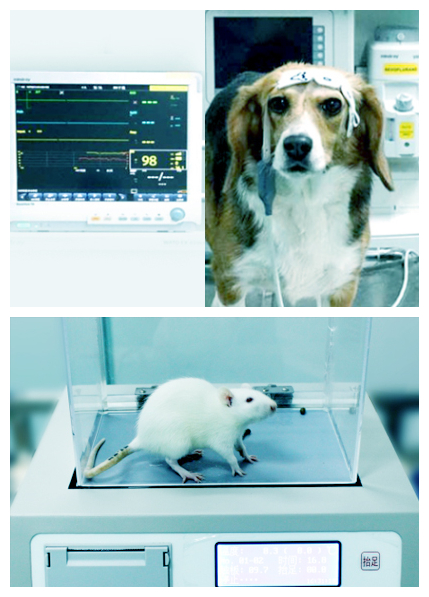
Core safety pharmacology study:
Central nervous system (CNS): functional observational battery (FOB), modified Irwin test;
Cardiovascular system: implanted telemetry, non-invasive vested telemetry, individualized corrected QT interval (QTc (IACF), QTbtb), arrhythmia analysis;
Safety pharmacological test of respiratory system: small animal whole body plethymography, large animal respiratory rate, sober large animal respiratory function flowmetry.
•
Supplemental safety pharmacological studies:
CNS: behavioral changes, learning and memory, and large animal non-invasive EEG telemetry;
Cardiovascular system: large animal echocardiography;
Respiratory system: airway resistance and pulmonary compliance, blood oxygen saturation and blood gas measurement, end-tidal carbon dioxide, ex vivo lavage of trachea of Guinea pig.



Business Contacts:028-60662528
E-mail:service@glpcd.com
+8628-60662518-8800
+8628-85154334-800
